NMPA Certifies Farsoon 3D Printed Tantalum Interspinal Fusion Cage
The company says the additively manufactured implants can be fully customized according to patients’ conditions, and the trabecular microstructure can achieve a high porosity of 68-78% to promote bone tissue and vessel fusion.
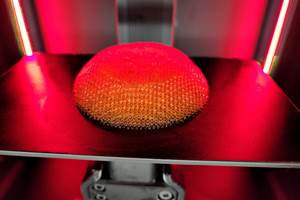
Tantalum interspinal fusion cage with porous structures designed for 3D printing. Photo Credit: Huaxiang Group
Huaxiang Group, a 3D printing solution provider with expertise in the medical industry, has received the category 3 medical device clearance from China’s National Medical Products Administration (NMPA) for its tantalum fusion cage additively manufactured on a Farsoon metal machine. The company says it is the first NMPA-approved tantalum orthopedic implant built by metal powder bed fusion technology in China.
Known as a specialized metal material with excellent biological inertness and compatibility, stable chemical properties and abrasion resistance, the tantalum metal material is well suited for medical implants. Since its first use in orthopedics in the 1940s, the tantalum has been used in many kinds of medical devices for nearly 80 years.
Due to the extremely high melting point (over 3,000°C), high density (16.6 g/cm3) and elastic modulus (185.7 GPa), the pure tantalum material can be quite challenging to process for use in the medical field. In the past 50 years, the conventional metal tantalum parts were manufactured through a complicated process — powder metallurgy or electron beam melting, deformation, welding and heat treatment.
With the application development of tantalum orthopedic products such as femoral head repair, cranial implant and joint prosthetics, the market has kept pushing the new designs and advanced manufacturing. It is said the tantalum implant with porous structure is proved in clinical practices to reduce stress while providing sufficient mechanical strength. It also encourages bone and vascular tissue growing into the porous structure. The chemical vapor deposition process was then developed for producing commercialized porous tantalum implants for medical use. However, with the limitation of the technology, it is only able to deliver standard end products, with a high manufacturing cost.
Compared to the previous manufacturing processes, Farsoon 3D printed tantalum porous interspinal fusion cage solutions developed by Huaxiang Group offers many advantages from design to manufacturing:
- The additively manufactured implants can be fully customized and produced according to patients’ conditions. The trabecular microstructure can achieve a high porosity of 68-78% to promote bone tissue and vessel fusion.
- The elastic modulus of the 3D printed tantalum implant is highly comparable to human cancellus and trabecular bone. It offers excellent stability, biomechanical compatibility and reduced stress-shielding.
- Precisely produced using the digital model, the implants can achieve high size accuracy, internal structure and designated roughness which only require minimal postprocessing.
- Excellent load-bearing capability. The additively manufactured implant is ready for immediate load-bearing with high toughness, good plasticity and fatigue resistance.
- Sustainable manufacturing with high material utilization.
- Improved efficiency with optimized manufacturing workflow.
- Reduced part lead time and cost.
- Read about Galactic Energy Space Technology’s first successful full-system test flight of its Welkin 50-ton reusable liquid oxygen (LOX)/kerosene engine. Several key components in the rocket engine are 3D printed by aerospace manufacturing service provider Falcontech using a metal laser powder bed fusion solution from Farsoon Technologies.
- Learn more about how Farsoon’s Quad-laser Flight technology on its HT1001P large-format platform offers an enhanced manufacturing speed to maximize production yield and lower manufacturing cost per part.
- Here’s information on how Farsoon’s FS621M Pro-4, FS621M Pro-6 and FS621M-U-4 systems are said to offer huge potential in aerospace applications by lowering operational cost and enabling true industrial-scale series manufacturing.
- This article details how Farsoon’s technology enabled an accelerated design-validation cycle of 80% faster compared to conventional manufacturing processes with the 3D printing of a large combustion chamber.
Related Content
Stryker Using Additive for Implants
Using its “AMagine” process, Stryker creates components with a titanium alloy that mimics bone.
Read MoreStratasys, CollPlant Unite Technologies for Industrial-Scale Bioprinting of Tissues, Organs
The joint development and commercialization agreement will initially focus on development of a bioprinting solution for CollPlant’s regenerative breast implants, addressing $2.6 billion market opportunity.
Read MoreMore Affordable Suture Anchors 3D Printed from PEKK: The Cool Parts Show #60
Selective laser sintering (SLS) of polyether ketone ketone (PEKK) is being used to produce medical implants that are more cost effective and perform better than their conventional counterparts. We highlight fasteners known as suture anchors in this episode of The Cool Parts Show.
Read MorePartners Improve Wheelchair Seats, Cushions Using 3D Printed Programmable Foam
The 3D printed programmable foam is said to enhance orthopedic seats and cushions, offering improved comfort and reliability for users.
Read MoreRead Next
3D Printing Brings Sustainability, Accessibility to Glass Manufacturing
Australian startup Maple Glass Printing has developed a process for extruding glass into artwork, lab implements and architectural elements. Along the way, the company has also found more efficient ways of recycling this material.
Read MoreTo Improve Performance of Compression Molded Composites, Add 3D Printed Preforms
9T Labs' Additive Fusion Technology enables the manufacture of composite structures with as much or as little reinforcement as is necessary, using 3D printed continuous fiber preforms to add strength just where needed.
Read MoreGE Additive Rebrands as Colibrium Additive
As part of the brand name transition, both the Concept Laser and Arcam EBM legacy brands will be retired.
Read More











.png;maxWidth=300;quality=90)